Virology Laboratory
Research Interest
-
Our lab research is focused on studying host-pathogen interactions in HIV infections and translating the basic findings in the development of novel anti-HIV retroviral drugs and latency reversal agents.
In a major advancement, research from my lab has significantly contributed to the development of maturation inhibitors as a novel class of anti-HIV retroviral drugs. Our research efforts have discovered and characterized the mechanism of action of a novel betulinic acid derivative anti-retroviral drug, Bevirimat (BVM). We have demonstrated that BVM analogs display potent anti-retroviral activity against major HIV-1 subtypes especially the most infectious HIV-1 subtype C. We have further identified and characterized resistant mutations which provide insights into the mechanism of resistance to these compounds. In a landmark study, published in Retrovirology, we have identified key polymorphisms in HIV subtype C, which regulate the sensitivity of the virus to the drugs. Our studies will be extremely beneficial in development of novel and broadly active therapies. In another study we focused on a different class of maturation inhibitor targeting HIV subtype B and C, the two most prevalent HIV-1 subtypes, and further clinical development of drugs appears promising.
For a complete cure of AIDS, it is important to target and reactivate latent HIV. In an important discovery, our group has identified and characterized a novel class of agents that reversed virus latency. HIV exploits specific proteins (also known as HIV-dependency factors) during its replication inside the cell. Potassium channels play a crucial role in the life cycle of several viruses. Our work demonstrated that potassium channels could serve as a safe therapeutic target for treatment of HIV/AIDS. Our group has established collaborations with industrial partners, such as DFH Pharma, USA, Pfizer, USA and Hetero Drugs, Hyderabad, India.
Recent Research Highlights
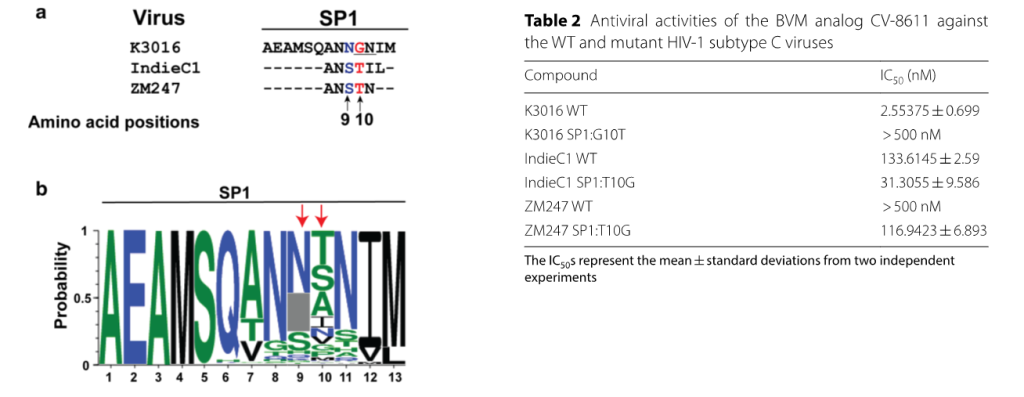
A single G10T polymorphism in HIV-1 subtype C Gag-SP1 regulates sensitivity to maturation inhibitors
Ghimire et al; Retrovirology (2021) 18:9
We have identified an association of a specific glycine at position 10 of Gag-SP1 with an MI susceptible phenotype of HIV-1 subtype C viruses. Our findings have highlighted that HIV-1 subtype C viruses, which were inherently resistant to BVM, may also be similarly predisposed to exhibit a significant degree of resistance to second-generation BVM analogs. Our work has strongly suggested that genetic differences between HIV-1 subtypes may produce variable MI sensitivity that needs to be considered in the development of novel, potent, broadly-active MIs.
Construction and characterization of a full-length, replication-competent and infectious enhanced green fluorescence protein-tagged HIV-1 subtype C molecular clone
Rai et al; Virology, (2022); 571; 34-38
HIV-1 subtype C virus accounts for nearly 50% of the total HIV infections globally. Despite this high prevalence, our understanding of subtype C specific infections remains limited due to lack of an in vitro model system. This is the first report of construction and characterization of a full-length and infectious EGFP-tagged HIV-1 subtype C molecular clone. The EGFP gene was inserted in-frame between the Nef and Env sequence in the HIV genome.The recombinant virus displayed expression of viral genes, infectivity and replication kinetics similar to the parental virus. VSV-G pseudotyping of the recombinant virus led to enhancement of HIV infection. The presence of the EGFP gene provides a rapid, easy and quantitative measure of HIV infection by flow cytometry and fluorescence microscopy. This clone will serve as an extremely beneficial tool to study HIV-1 subtype C specific infections.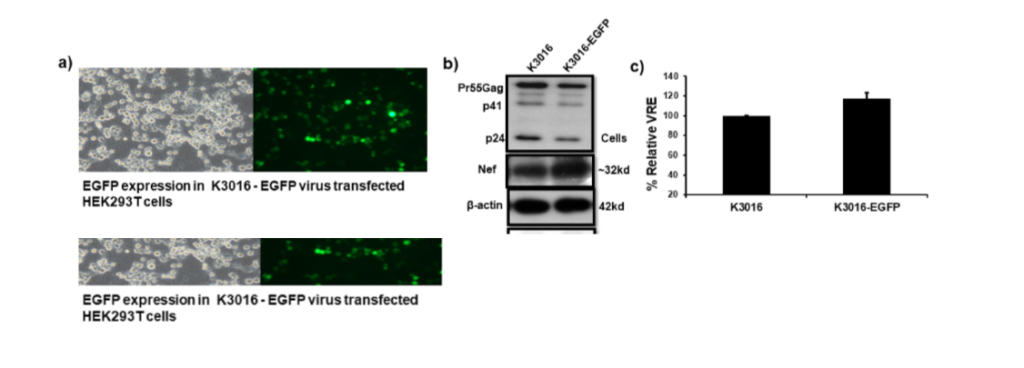
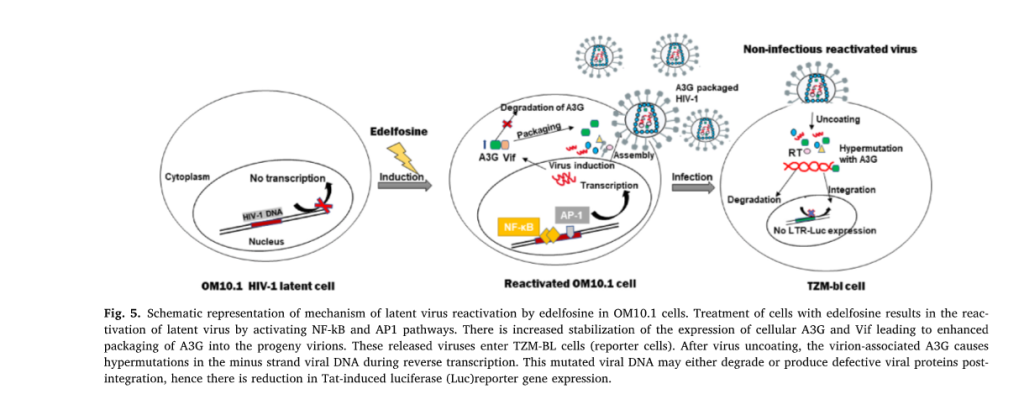
Edelfosine reactivates latent HIV-1 reservoirs in myeloid cells through
activation of NF-κB and AP1 pathway.
Rai et al; Virology (2022) 574; 57-64
The persistence of latent HIV-1 reservoirs in cells presents a formidable challenge towards a complete HIV cure. Edelfosine is an FDA-approved investigational, anti-neoplastic drug. In this study, we aimed to investigate its role as a HIV-1 Latency Reversal Agent (LRA) using latency model cell lines. Our findings demonstrated that edelfosine reactivated latent HIV-1 viruses in myeloid cells in a dose and time-dependent manner. The mechanism of reactivation by edelfosine involved the activation of NF-κB and AP1 pathways in these cells. The reactivated virus was non-infectious. Delineating the mechanism of non-infectious virus production revealed an increased stabilization of cellular APOBEC3G protein as well as its enhanced incorporation into the released viruses. Thus, our study demonstrated for the first time an additional role of edelfosine in reactivation of latent HIV-1 and production of non-infectious virus. Our results have paved the way for repurposing of edelfosine as a novel HIV-1 latency reversal agent.
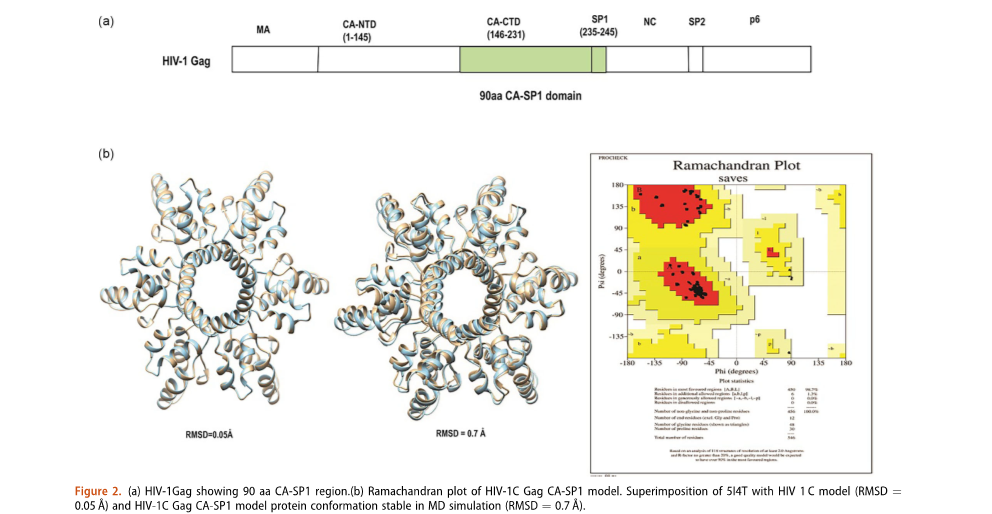
Construction of a HIV-1 subtype C 3D model using homology modeling and in-silico docking, molecular dynamics simulation, and MM-GBSA calculation of second-generation HIV-1 maturation inhibitor(s)
Yuvraj KC et al; J Biomol Struct Dyn. ; 2023 Jul 24:1-10.
We have developed a 3D model of HIV-1C Gag CA-SP1 region using protein homology modeling with HIV-1 subtype B(514T) as a template. The HIV-1 C homology model generated was extensively validated using several online tools and served as a template to perform molecular docking studies with eight well-characterized MIs. The docked complex of HIV-1C and all nine MIs was subjected to molecular dynamics simulation for 100 ns using AMBER and binding free energy calculations were done using MM-GBSA.
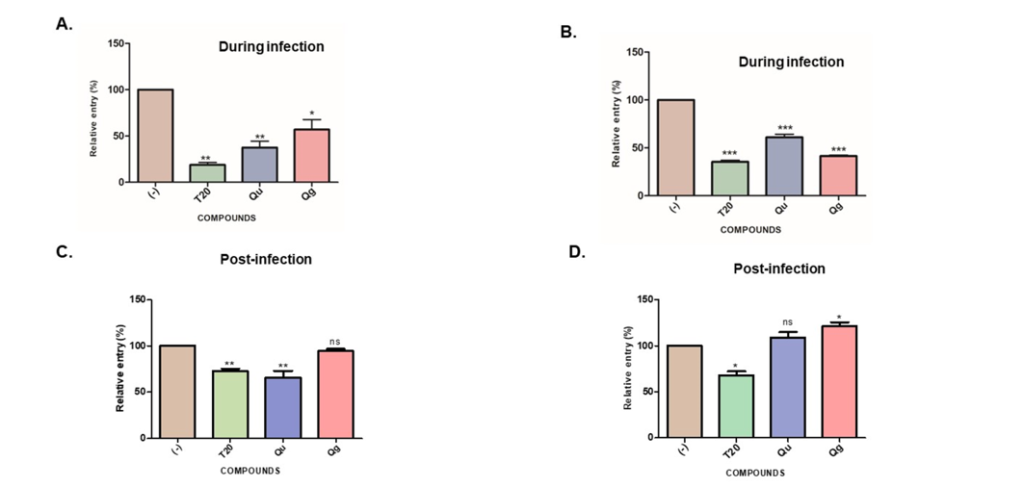
Microwave synthesized hydrophilic 4-oxo-2-phenylquinazoline-3(4H)-carboximidamide and –carboxamide as small anti-HIV-1 molecules.
Aradhana Singh et al; Chemistry Select; 2024
Opportunistic infections like tuberculosis (TB) are prevalent in HIV-infected individuals. In an approach to introducing an industrial-scale production of agents having both antiviral and antibacterial properties, a set of hydrophilic 2,3-substituted quinazolinones, 4-oxo-2-phenylquinazoline-3(4H)-carboximidamide (Qg) and 4-oxo-2-phenylquinazoline-3(4H)-carboxamide (Qu), were afforded in encouraging yields under microwave synthesis. The study identifies the antiviral properties of the two benzo-fused pyrimidine derivatives, which were earlier reported to have antitubercular activity.
Patent Obtained:
Co-inventor on patent (# 454199) granted on 25/09/2023 – Title- MESO-TRIS-CARBOXY PHENYL PORPHYRIN- FULLERENE ADDUCTS AS ANTI-HIV AGENTS.
Current Lab members

PhD Student
yuvraj.bhu2009@gmail.com
PhD project: Targeting HIV-1 maturation and latency using novel compounds

PhD student
aradhanasingh1401@gmail.com
PhD project: Studies on development of novel anti-HIV compounds and gene expression profiling of HIV-1 subtype B and C

PhD student
Vidyajan2023@gmail.com
Recent Article: A cure for HIV: Where do we stand?
Alumni

Postdoctoral Research Associate
Department of Microbiology and Immunology,
SUNY University at Buffalo, Buffalo, New York, USA
timilsinau@gmail.com

Postdoctoral Research Associate
Department of Biochemistry and Molecular Biophysics,
Washington University in St. Louis, Missouri, USA
nmishra@wustl.edu

Researcher
Genestore India Services Private Limited,
Gurgaon, India.
ravi.d@genestore.in

Research Fellow
Infectious Disease Unit, Massachusetts General Hospital,
Harvard Medical School, Boston, MA, USA
dibya.ghimire4@gmail.com

University of Minnesota
Twin cities Campus
Madhu@umn.edu
Lab activities: